센터소개About Us
연구현황 | Research
Proteogenomic landscape of human pancreatic ductal adenocarcinoma in an Asian population reveals tumor cell-enriched and immune-rich subtypes
Hyeon D.Y.; Nam D.; Han Y.; Kim D.K.; Kim G.; Kim D.; Bae J.; Back S.; Mun D.-G.; Madar I.H.; Lee H.; Kim S.-J.; Kim H.; Hyun S.; Kim C.R.; Choi S.A.; Kim Y.R.; Jeong J.; Jeon S.; Choo Y.W.; Lee K.B.; Kwon W.; Choi S.; Goo T.; Park T.; Suh Y.-A.; Kim H.; Ku J.-L.; Kim M.-S.; Paek E.; Park D.; Jung K.; Baek S.H.; Jang J.-Y, Hwang D.; Lee S.-W.; Nature Cancer, 4, 290-307, 2023

We report a proteogenomic analysis of pancreatic ductal adenocarcinoma (PDAC). Mutation–phosphorylation correlations identified signaling pathways associated with somatic mutations in significantly mutated genes. Messenger RNA–protein abundance correlations revealed potential prognostic biomarkers correlated with patient survival. Integrated clustering of mRNA, protein and phosphorylation data identified six PDAC subtypes. Cellular pathways represented by mRNA and protein signatures, defining the subtypes and compositions of cell types in the subtypes, characterized them as classical p rogenitor (TS1), squamous (TS2–4), immunogenic progenitor (IS1) and exocrine-like (IS2) subtypes. Compared with the mRNA data, protein and phosphorylation data further classified the squamous subtypes into activated stroma-enriched (TS2), invasive (TS3) and invasive-proliferative (TS4) squamous subtypes. ...
Publication List
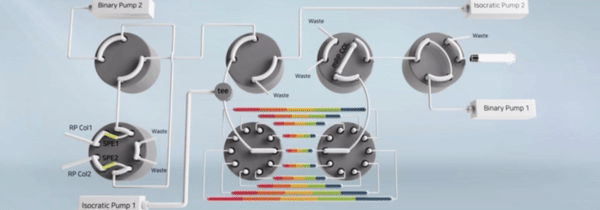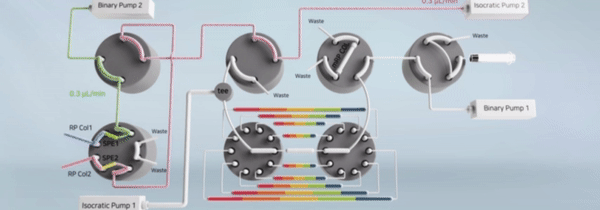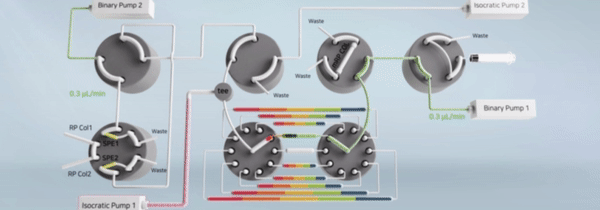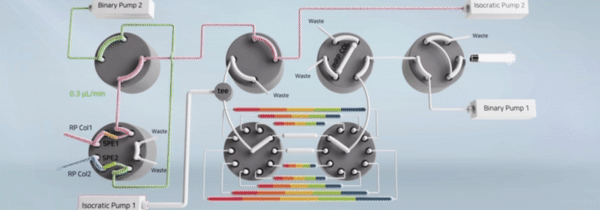
"비연속적 시료 분획 및 통합유닛과 이를 구비한 이중 온라인 다기능 액체크로마토그래피 장치" 등록일 2024년 5월 14일